Dementia conditions are very common, presenting in around 7% of people over the age of 65. Despite this, accuracy of dementia diagnosis can be poor, with the causes of dementia often being misdiagnosed. For example, although dementia with Lewy bodies (DLB) is one of the most common causes of dementia in the UK, approximately 20% of people with DLB receive the wrong diagnosis. DLB is a type of progressive dementia, associated with clinical symptoms such as hallucinations, delusions, sleep disturbances, memory problems, movement issues, and confusion. Although DLB is not as well-known Alzheimer’s, it has a huge impact on people’s lives. The condition became better known to the public in 2016 when actor Robin Williams was revealed to have had DLB.
Compared with other parts of the UK, significantly more cases of DLB are reported in North East England. This is not thought to be due to people in the North East being more likely to develop DLB. It likely reflects Newcastle University’s long history of DLB research and the effect this has on medical education training in the area. This increased awareness may lead to an improved rate of disease detection.
Research directly impacts clinical approaches to diagnosis of conditions, with clinicians constantly adapting and growing along with improved scientific knowledge. It is therefore crucial to ensure that researchers employ cutting-edge techniques and recruit appropriate participants for these studies. For example, when we are testing a drug therapy on people with Alzheimer’s disease, we want to be confident that our participants have the correct diagnosis. If 20% of these people have been misdiagnosed and really have DLB, this may have a major negative effect on the trial. It is pivotal that researchers use the best possible evidence-based diagnostic systems.
How can researchers more accurately diagnose people with dementia?
To avoid recruitment of the wrong people, we should use a standardised set of rules for disease diagnosis. Recently, researchers at Newcastle University published such criteria for the early stages of DLB when patients are experiencing milder symptoms, referred to as mild cognitive impairment with Lewy bodies (MCI-LB). Mild cognitive impairment means that an individual shows impairments in at least one cognitive function, such as memory, attention, language abilities and complex reasoning, but can maintain their independence, such as their abilities to carry out everyday tasks. It is very helpful for researchers to recruit people at these early stages, so that the patients can be observed over a long period, and we can find out more about the progression of the disease, such as how long it typically takes for a person to transition from MCI-LB to the dementia stages of DLB. By characterising these early stages, clinicians may be able to detect people at-risk of developing dementia.
The research criteria give ‘essential’, ‘core’, and ‘supportive’ clinical features of MCI-LB. A diagnosis of MCI-LB arises when an individual presents with a discrete combination of these features, which are listed in the table. Clinical features are symptoms that may occur over the course of the condition. In people with MCI-LB, mild cognitive impairment is deemed the essential clinical feature.
What are the clinical features of Mild Cognitive Impairment due to Lewy Bodies?
DLB has a number of core clinical features, including presence of visual hallucinations, movement problems and sleep disturbances. However, in the research criteria for MCI-LB, only one of these features has to occur to indicate the person may have the condition. Supportive features such as repeated falls or excessive sleepiness, may enhance the researcher’s confidence that they are accurately diagnosing the individual. These supportive features are particularly useful for diagnosis when they persist over time or occur in combination with each other.
Proposed biomarkers are also suggested in the criteria. These are physical or biological changes that researchers can see when they do scans or recordings of brain activity in patients. They can be very useful for identifying and distinguishing the condition from other dementia-causing diseases. This is because biomarkers provide an objective, measurable way to characterise the condition, whereas clinical symptoms are subjective. A full list of the clinical features and biomarkers is given in the table below.
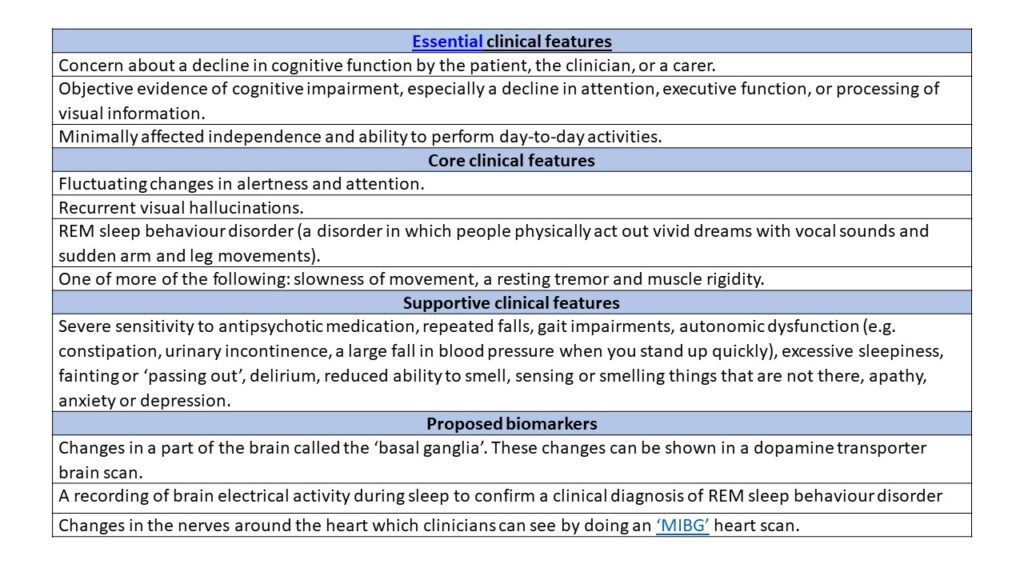
REM, rapid eye movement; MIBG, metaiodobenzylguanidine.
According to the research criteria, an individual can be diagnosed as having either probable MCI-LB or possible MCI-LB. For either, the person must have mild cognitive impairment, defined as the presence of all the essential features shown in the table.
Probable MCI-LB can be diagnosed if a person has two or more core clinical features, or one core clinical feature with at least one proposed biomarker. Possible MCI-LB can be diagnosed if patients have either one core clinical feature with no biomarkers, or one or more of the proposed biomarkers but no core clinical features. This distinction is useful as some DLB characteristics hold more weight in diagnosis due to increased evidence backing up their specificity for the condition. Also, there are certain cases in which dementia patients may have multiple conditions causing their symptoms. For example, autopsy studies have found that up to half of those diagnosed with Alzheimer’s disease may also have unsuspected DLB pathology. Therefore, if a person is showing ‘atypical’ signs of DLB, it can be difficult to confidently diagnose the individual with just one condition.
This new method for diagnosing MCI-LB in research is an exciting step forward in this field. At the moment, these criteria are only intended for use in research. More validation is required before they will be implemented into clinical practice. As research is the foundation of clinical growth and development, it is vital that the data collected in these studies are accurate and applicable to this patient population. Scientists can now use this method of MCI-LB diagnosis to collect more valuable, accurate study data. If these criteria are one day used clinically, they may allow earlier treatment and better management and care for people with DLB, before symptoms become too debilitating. Clinicians could streamline care and anticipate treatment options known to be effective in DLB. In addition, early diagnosis would help patients and their friends/families to plan ahead and make important lifestyle changes that may alleviate aspects of the condition. It is my hope that these criteria will one day provide clinicians with the tools necessary to improve the quality of life of the millions of people living with DLB across the globe.

Sammy Waite is a second year PhD student at the Newcastle NIHR Biomedical Research Centre in Newcastle upon Tyne, UK. She carries out research investigating ageing-related changes in muscle and physical function in individuals in the early stages of dementia with Lewy bodies and Alzheimer’s disease.
Twitter: @waite_sammy




