Standing in a half empty room – the majority being supportive colleagues – at the last session on the last day (referred to as the “grave-yard shift”) at the 2014 Alzheimer’s Association International Conference, never did I think medical science could evolve so quickly – where a blood test for Alzheimer’s disease is no longer described as being “on the horizon” but is staring us directly in the face. Of course, in 2014, optimistic correlations of peripheral proteins in the blood with clinical symptoms were the order-of-the-day. These early efforts generated enthusiastic interest and persuaded large research cohorts to include standardised plasma collections in their protocols. Now, it has certainly paid-off.
But, 2014 was not the start of this process. My mentors and PhD supervisors at the time, Dr. Abdul Hye and Professor Simon Lovestone, had already hinted that a blood biomarker could be a realistic possibility in a series of publications between 2006-2010. They described that proteins linked to biological processes like inflammation or immune system are changed in the blood of Alzheimer’s disease patients. Importantly, these proteins are closely linked to Alzheimer’s pathology found at post-mortem and were later found to be genetic risk factors for Alzheimer’s (e.g., clusterin). Although these reported blood tests did not have the required accuracy needed to be clinically relevant, they generated the necessary interest in that propelled this area of Neuroscience to where it is now.
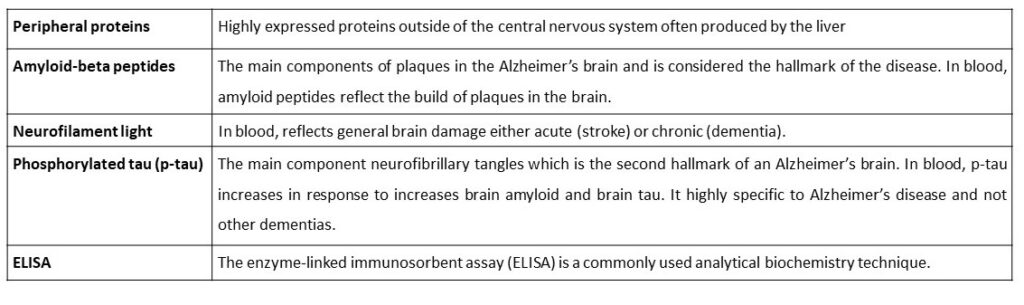
So, what has changed? Well, certainly the intended targets have not. The “new” generation of blood biomarkers we talk about today are the classical candidates we have known about for many decades in the brains and cerebrospinal fluid (CSF) of Alzheimer’s disease patients; amyloid-beta peptides, neurofilament light and phosphorylated tau (p-tau; see Table for definitions). I believe there are three fundamental changes responsible for this rapid development of a blood test for Alzheimer’s. Firstly, we have recently begun to change the way we think about Alzheimer’s disease. In 2018, the National Institute on Aging and Alzheimer’s Association (NIA-AA) defines Alzheimer’s disease as a biological construct – simply meaning, you must have evidence of Alzheimer’s disease pathology in the brain related to the observed cognitive symptoms for the disease to be called Alzheimer’s. Secondly, there are now large well-characterised research cohorts (e.g., Swedish BioFINDER, the Alzheimer’s Disease Neuroimaging Initiative and Translational Biomarkers in Aging and Dementia, to name but a few) which have scrupulous details about their participants biology e.g., molecular imaging for tau and amyloid in the brain. This gives us the best chance to see if our blood tests really reflect brain pathology. Lastly, our technology. These proteins exist at femtomolar concentrations (10-15 molecules per litre) in the blood and previously we could only detect them in extreme neurological cases or, in case of p-tau, not at all. Owing to the advancements in mass spectrometry and ultra-sensitive versions of the good-old ELISA (as defined in the Table), we can detect these proteins in all people, of any age.
What’s the big deal? Why is a blood test needed? If you know anything about Alzheimer’s disease you may ask several questions. Surely it is not hard for an experienced clinician to tell the difference between someone who has Alzheimer’s compared to someone who is ageing in a healthy manner? Why do we need such a test if we have no treatment? And can’t we already visualise these changes in the brain by advanced brain imaging or spinal fluid tests? These would be very valid and important questions. There are two intended purposes a blood test for Alzheimer’s disease. Firstly, the “case versus control” design is restricted to research and in reality, a blood test would be of value to a clinician to rapidly indicate if the cognitive decline being exhibited by a patient is a result of Alzheimer’s or should they undergo more extensive testing for other rarer types of dementia or even something unrelated to dementia. This would not only improve the confidence of the diagnosis for the patient and their family but also improve the confidence in administrating symptomatic treatment that is available for Alzheimer’s disease. Second, there is an extremely large effort to find the elusive disease-modifying therapy for Alzheimer’s and there have been some high-profile failures in recent years. In order for a drug to work, the individuals in these trials need to have the intended pathology (in this case amyloid or tau) – in recent years, this has been difficult to achieve given the high cost of molecular imaging and lumbar puncture to obtain spinal fluid being quite an unpleasant experience. A blood biomarker is the only way to engage a large enough population, sometimes those without symptoms, so that the correct people are recruited into these trails and definitely test if these drugs have a benefit to patients.
So, what are the next steps? Well, a blood test for neurofilament light , a protein indicative for non-specific brain neurodegeneration, has momentously been implemented into clinical routine here in Sweden, led by Prof. Henrik Zetterberg and Prof. Kaj Blennow and can now be requested by clinicians all around the country – other countries will rapidly follow suit. Spinal fluid collection by lumbar puncture is relativity common in the neurological examination of patients in Sweden, so the greatest benefit of such a test will be felt in the United Kingdom or the United States where a spinal fluid test is often avoided, due to its perceived invasiveness. A specific blood test for Alzheimer’s disease, p-tau, has received a lot of attention this year, with some high-profile publications in the Lancet, Nature and JAMA. Already, international efforts are underway to validate these p-tau blood for clinical use with the field unanimously convinced of value to the clinical examination of dementia patients and the potential benefit to aid therapeutic trials.
All these biomarker developments have happened in parallel with the development of novel therapeutics. Within the next few months, we will know if the Food and Drug Administration (FDA) will approve the clinical use of the very first disease-modifying drug for Alzheimer’s, an anti-amyloid antibody which aims to remove disease pathology from the brains of patients with early or mild Alzheimer’s disease. A requirement to be prescribed such a drug will most likely be that the clinical diagnosis is supported by a positive Alzheimer’s biological test. As discussed, a spinal fluid tests or a molecule imaging scans would be too problematic if these drugs become widely available. This is where a simple blood test would play a key role – at the very least serve as a triage tool. In the era of personalised medicine, maybe a simple blood test could help selecting the right patient for the right drug and at the right dose. Exactly how this will work in real life, I do not know, but we now have the tools at hand to aid these therapeutic and clinical decisions. Only one detail is missing: an approved drug! But we may have it sooner than anyone thought just a few years ago. To me the future looks promising, particularly in combating this disease that affects so many people and it is extremely exciting being at the forefront of these scientific advances. I think I will have a significant amount of work to do in the coming months and years.

Dr. Nicholas Ashton is a Neuroscientist and Assistant Professor at the Department of Psychiatry and Neurochemistry, University of Gothenburg, Sweden. Dr. Ashton holds a BSc in Forensic Chemistry, an MSc in Clinical Neuroscience and received his PhD from the department of King’s College London in 2017. His research predominately focuses on discovering new ways to detect Alzheimer’s disease and related disorders, particularly blood and cerebrospinal fluid.
Email: nicholas.ashton@gu.se




